| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
원료 ≫ 산미료 chelate : 구연산 citric acid, 구연산나트륨 구연산 citric acid : 산미료 - GM 미생물 : 구연산 생산 - chelate : 구연산, 구연산나트륨 - 봉쇄제 Chelating agent  칼슘은 좋은 킬레이트제로 금속이온을 쉽게 녹게 한다. 보일러 증발기 등의 스케일제거에도 좋으며 물의 경도는 낮추는데도 유용하다. 그래서 구연산은 욕실이나 주방세제에 활성성분으로 사용되기도 한다. 6% 정도의 용액은 묵은 때의 제거에 효과적이다. 녹을 제거하는데도 유용하고, 삼푸에서는 왁스를 녹이는 기능을 한다. 심지어 구연산은 1940년대 원자탄을 개발하기 위한 맨하탄 프로젝트에서 란탄계열의 원자를 분리하는 목적으로도 사용되었다. 그러다 1950년도에는 보다 효과적인 EDTA에 의해 대체되었다 Citric acid is an excellent chelating agent, binding metals by making them soluble. It is used to remove and discourage the buildup of limescale from boilers and evaporators. It can be used to treat water, which makes it useful in improving the effectiveness of soaps and laundry detergents. By chelating the metals in hard water, it lets these cleaners produce foam and work better without need for water softening. Citric acid is the active ingredient in some bathroom and kitchen cleaning solutions. A solution with a six percent concentration of citric acid will remove hard water stains from glass without scrubbing. In industry, it is used to dissolve rust from steel. Citric acid can be used in shampoo to wash out wax and coloring from the hair. Illustrative of its chelating abilities, citric acid was the first successful eluant used for total ion-exchange separation of the lanthanides, during the Manhattan Project in the 1940s. In the 1950s, it was replaced by the far more efficient EDTA. 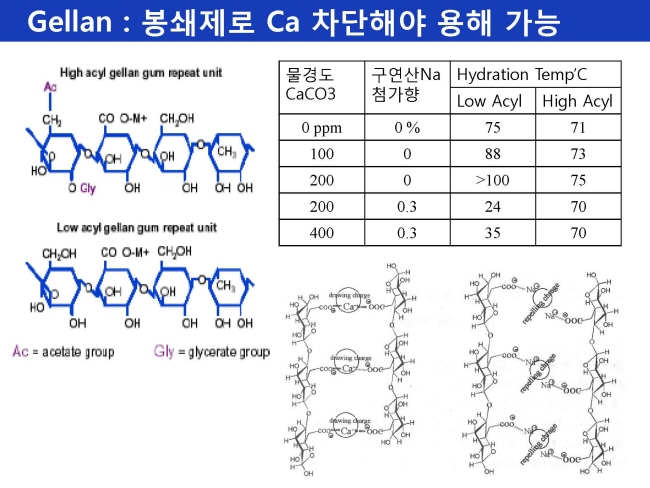  |
||||
|
|
|||