증점제 : 셀룰로스계 CMC
물성 ≫ 증점 ≫ 증점제 종류
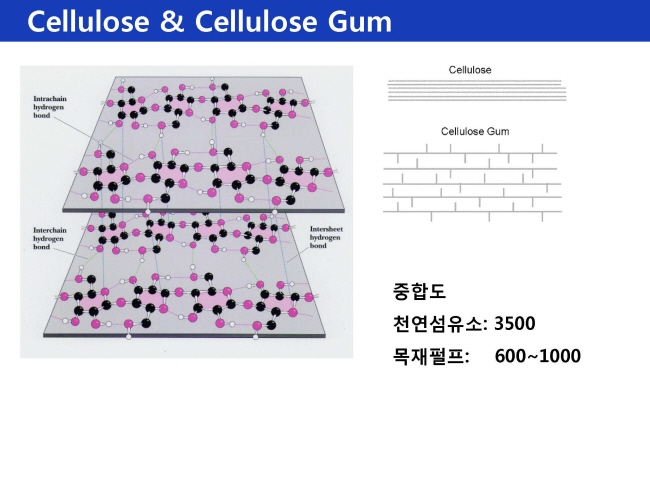
셀룰로스계 Cellulose 
cellulose
- 포도당 결합 : 아밀로스, 아밀로펙틴
- 셀룰로오스를 분해하기 힘든 이유
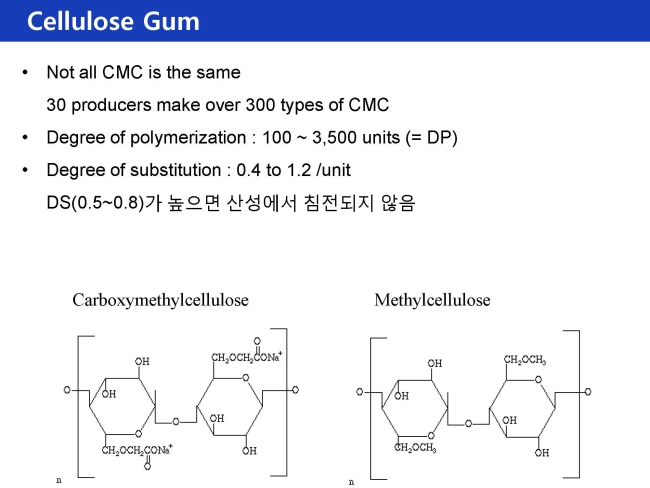
- cellulose gum : Methyl cellulose
Cellulose = 겹겹이 쌓인구조로 비집고 들어갈 틈이없다.
Cellulose gum = Side chain 있어서 수화 가능
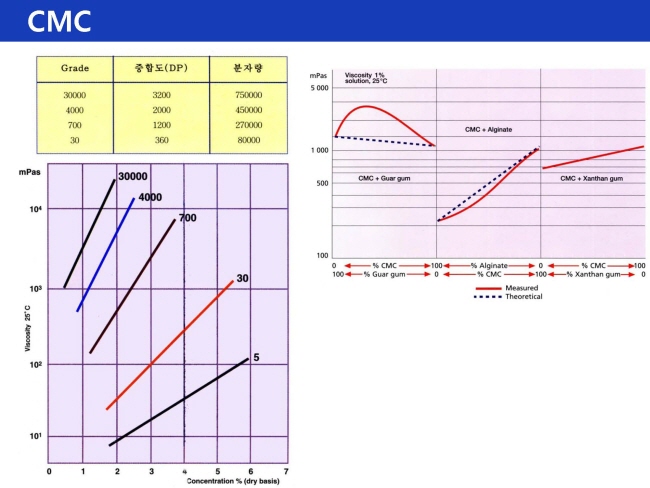
- DP : 체인 길이에 딸라
Fat holdout
- Methyl celllulose
- Hydroxypropylcellulose
- Methylhydroxypropylcellulose


Carboxymethyl cellulose (CMC) (cellulose gum) is prepared from cotton linters or wood pulp by soaking the raw material in aqueous sodium hydroxide and then introducing monochloroacetic acid. The result is the introduction of carboxymethyl ether groups where there had been hydroxyl groups previously. The reacted cellulose is then purified, washed, and dried.
Methylcellulose (MC) and methylhydroxypropylcellulose (MHPC) are prepared in a manner similar to that of CMC, except that the reagents used are methyl chloride for MC and a mixture of methyl chloride and propylene oxide for MHPC. Hydroxypropylcellulose (HPC) is also prepared in a similar manner, except that the reagent used is propylene oxide.
while CMC is anionic (and is sold in the sodium salt form), the other three cellulose-based hydrocolloids (MC, HPC, and MHPC) are non-ionic. In practical terms, this makes MC, HPC, and MHPC relatively insensitive to electrolytes, while the viscosity of CMC decreases dramatically if it is not hydrated in water prior to exposure to ionic substances such as salts and acids.
As a class, the cellulose derivative hydrocolloids are optically transparent in solution, while most other gums are not. They make pseudoplastic solutions (i.e., exhibit instantaneous and reversible shear thinning behavior), provided that the substitution is evenly distributed along the molecule. Where they differ
is in the way they respond to increases in solution temperature. A CMC solution thins when heated and thickens again when cooled, which is typical behavior for most gums. MC and MHPC solutions gel if heated to their incipient gelation temperature (IGT) and revert to the liquid state upon cooling. An HPC
solution precipitates if heated to its cloud point but redissolves when cooled to room temperature. These three very different behaviors result from the differences in the type of substitutions linked on the cellulose backbone. In MC, HPC, and MHPC, the DS controls when the IGT or cloud point occurs and the ultimate gel strength. Generally, as the hydroxypropyl DS increases, the IGT increases while the gel strength decreases (softer gel). The IGT is usually
50–90°C (122–194°F). If MC and MHPC are heated sufficiently beyond the IGT, they precipitate just as HPC does. HPC does not have a “gelled”
state like MC and MHPC. It goes from hydrated to precipitated when its solution temperature is raised sufficiently. As the DS of CMC increases, the tolerance to electrolytes increases and the probability of thixotropic behavior decreases.
작년(2015년 2월25일) 네이처(Nature)지에 유화제의 위험성이 밝혀졌다는 논문이 발표되었다. 미국 조지아 주립대학 생의학연구소의 앤드루 지워츠 박사는 유화제가 장박테리아 구성에 변화를 일으켜 염증 촉진 박테리아는 증가시키고 염증 억제 박테리아는 감소시킨다는 쥐실험 결과를 발표한 것이다. 실험에서 사용한 유화제인 폴리소르베이트-80(polysorbate-80) 또는 CMC(carboxymethylcellulose) 였다. 실험에 따르면 이것을 섭취한 쥐들이 쥐들이 모두 장박테리아의 구성에 변화가 나타나면서 플라겔린(flagelin)과 다당체(lipopolysaccharide)를 많이 만드는 박테리아들이 증가했다는 것이다
식품에는 10ppm 이하, 0.5ppm 이하 같이 농도를 기준으로 하는 경우가 많다. 이것은 일반적인 식품의 섭취 상황을 고려하여 농도이다. 그런데 섭취량을 고려하지 않고 무작정 농도를 기준으로 하는 어리석은 실험도 꽤 있다. 만약에 사람이 CMC(Carboxymethyl cellulose, 증점제) 1% 제품을 먹인 실험을 하였다면. 통상 사용량보다 많지만 그 농도를 가지고 문제를 삼기는 힘들다. 그런데, 20g짜리 쥐에게 1% 짜리를 매일 5ml를 먹이면 문제가 엄청나다. 성인 기준으로 매일 150g을 먹인 셈이 되니까 말이다. 이런 실험은 독성을 논하기 전에 실험의 기본이 안 된 것이다. 어떠한 성분이라도 매일 150g을 먹인다면 탈이 나기 쉬운 양이기 때문이다. 그런데 이런 실험이 가장 권위 있다는 Nature에 실리기도 하니 불량지식과의 투쟁은 고단할 수밖에 없다.
|
|
Network ≫ 두뇌, 마음, 욕구
뇌의 작동원리, 지도원리
감각 기관
보상시스템
- 학습 파페츠
- 목적 동기 부여 회로
식욕 mechanism
- Food Pleasure
file.txt
file.txt
|