|
암 ≫ 오해 ≫ 발암물질
1,3-Butadiene 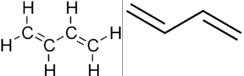
- 위키백과사전 link
Most butadiene is polymerized to produce synthetic rubber. While polybutadiene itself is a very soft, almost liquid material, polymers prepared from mixtures of butadiene with styrene or acrylonitrile, such as ABS, are both tough and elastic. Styrene-butadiene rubber is the material most commonly used for the production of automobile tires.
Smaller amounts of butadiene are used to make the nylon intermediate, adiponitrile, by the addition of a molecule of hydrogen cyanide to each of the double bonds in a process called hydrocyanation developed by DuPont. Other synthetic rubber materials such as chloroprene, and the solvent sulfolane are also manufactured from butadiene. Butadiene is used in the industrial production of 4-vinylcyclohexene via a Diels Alder dimerization reaction[6] and the vinylcyclohexene is a common impurity found in butadiene upon storage. Cyclooctadiene and cyclododecatriene are produced via nickel- or titanium-catalyzed dimerization and trimerization reactions, respectively. Butadiene is also useful in the synthesis of cycloalkanes and cycloalkenes, as it reacts with double and triple carbon-carbon bonds through the Diels-Alder reaction.
|
|