|
단백질 ≫ 아미노산 ≫ 아미노산 합성
세린 Serine
- Gluamic acid, glutamine
- Aspartic acid, asparagine
- serine, threonine, glycine, valine
- alanine, leucine, isoleucine
- lysine, arginine, histidine, proline
- methionine, Cysteine
- Tyrosine, Phenylalanie, Tryptophan
대부분의 단백질 속에 존재하며, 명주[絹] 의 단백질인 세리신에 특히 풍부, 유단백 속에는 인산에스테르의 형태로 존재하며
D-세린의 경우 누에의 혈액 등에 존재
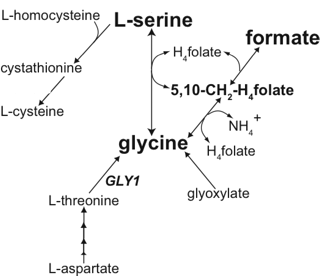
생체 내에서는 글리신과 함께 대사계의 매체적 역할을 하며, 시스틴과 메티오닌의 상호변환에 관여
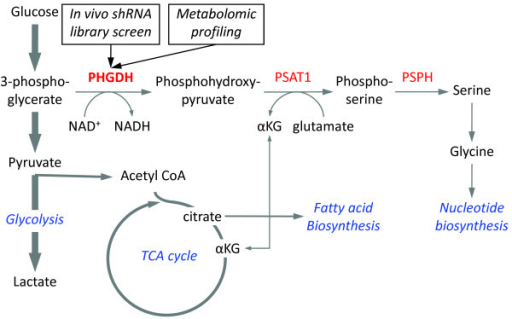
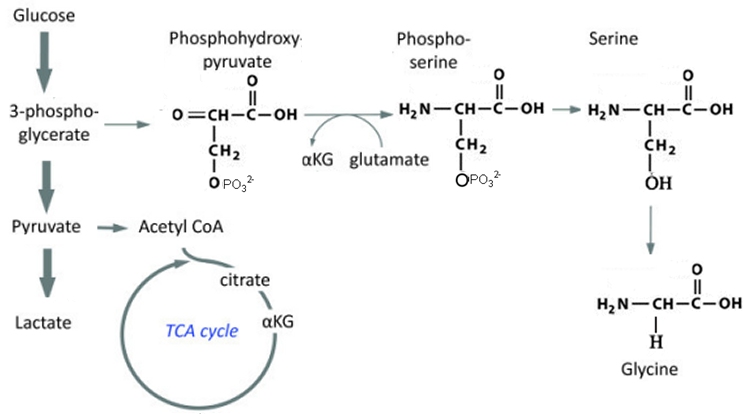
세린은 시스테인, 퓨린과 피리미딘의 생합성에 참여한다는 점에서 신진 대사에서 중요하다. 박테리아에서 트립토판뿐만 아니라 글리신과 시스테인을 포함한 여러 아미노산의 전구체다. 그것은 스핑고리피드(sphingolipid)과 엽산(folate)을 포함한 수많은 다른 대사 물질의 전구체이기도 하다. 세린은 많은 효소의 촉매 작용에 중요한 역할을 한다. 그것은 chymotrypsin, trypsin 및 다른 많은 효소의 활성 부위에서 존재한다. 신경가스 및 살충제에 중에는 아세틸콜린에스테라제 활성부위의 세린 잔기와 결합하여 작용하는 것이 많다.
세린은 의약품, 화장품, 영양보조식품용 등에 이용되고 있다. 의약품으로 가장 많이 쓰이며 저단백혈증, 저영양상태 등에 있어 아미노산보급에 이용된다, D형 세린도 여러 가지 기능을 갖고 있는 것이 알려져 왔다. 세린의 기능에는 신경전달물질의 아세틸콜린을 만들어 내어 글리신과 함께 중추신경의 존속을 촉진하는 작용이 있다. 또, 최근 연구에서는 뇌 안에서의 세린생합성이 주요 흥분성전달물질 글루탐산의 NMDA형 수용체의 활성화인자인 D-세린의 유지와 동 수용체의 기능에 불가결하다는 것이 해명되었다. 그리고 뇌 안에서의 세린부족이 통합실조증 증상에 관여하고 있는 것도 밝혀졌다. 게다가 해마나 푸키네세포의 중추신경제포 생존유지와 수상돌기 등의 형태형성은 성성교세포가 적극적으로 세포외에 분비하고 있는 세린에 의존하고 있는 것도 알려졌다.
화장품 분야에서는 세린이 세포막을 구성하는 인지질의 포스파티딜세린의 구성성분으로써 중요하며, 체내에서 시스테인으로 변환되는 것 때문에 미백이나 모발용 크림이나 로션 등에 첨가되고 있다. 또 식품에서는 감칠맛 등을 부여 맛을 조정하는 첨가제로써 이용되고 있다.
Cysteine synthesis from serine.
Cystathionine beta synthase catalyzes the upper reaction and cystathionine gamma-lyase catalyzes the lower reaction.
Serine is important in metabolism in that it participates in the biosynthesis of purines and pyrimidines.
It is the precursor to several amino acids including glycine and cysteine, and tryptophan in bacteria.
It is also the precursor to numerous other metabolites, including sphingolipids and folate, which is the principal donor of one-carbon fragments in biosynthesis.
Structural role
Serine plays an important role in the catalytic function of many enzymes. It has been shown to occur in the active sites of chymotrypsin, trypsin, and many other enzymes. The so-called nerve gases and many substances used in insecticides have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely.
As a constituent (residue) of proteins, its side chain can undergo O-linked glycosylation, which may be functionally related to[clarification needed] diabetes.
It is one of three amino acid residues that are commonly phosphorylated by kinases during cell signaling in eukaryotes. Phosphorylated serine residues are often referred to as phosphoserine.
Serine proteases are a common type of protease.
Signaling
D-Serine, synthesized in the brain by serine racemase from L-serine (its enantiomer), serves as both a neurotransmitter and a gliotransmitter by activating NMDA receptors, making them able to open if they then also bind glutamate. D-serine is a potent agonist at the glycine site of the NMDA-type glutamate receptor. For the receptor to open, glutamate and either glycine or D-serine must bind to it. In fact, D-serine is a more potent agonist at the glycine site on the NMDAR than glycine itself. D-serine was only thought to exist in bacteria until relatively recently; it was the second D amino acid discovered to naturally exist in humans, present as a signalling molecule in the brain, soon after the discovery of D-aspartate. Had D amino acids been discovered in humans sooner, the glycine site on the NMDA receptor might instead be named the D-serine site. [8]

|
|