|
식품 ≫ 단백질 ≫ 계란
계란 : 난단백, 응고
단백질 공급원
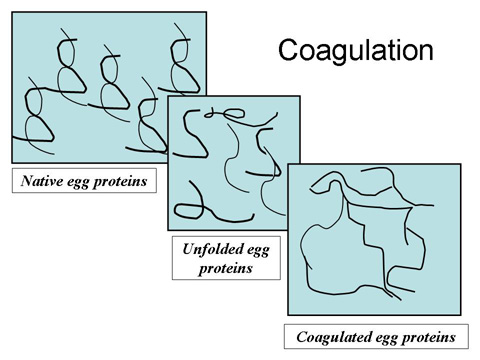
단백질응고
- 계란, 난백
- 단백질 unfolding
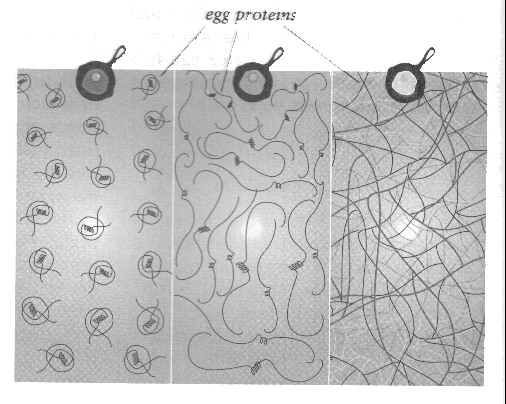




Ovalbumen
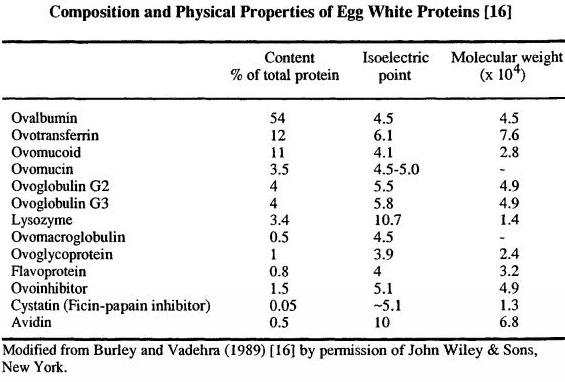
• The predominant protein in albumen (54% of albumen).
• Classified as a phosphoglycoprotein since carbohydrate and phosphate moieties are attached to the polypeptide.
• MW of about 45,000, and made up of 3 components, A1, A2 and A3, which differ in phosphorous content.
• Ovoalbumen A1 has two phosphate per molecule, A2 has one, and A3 has none.
• Ovoalbumen in solution is readily denatured and coagulated by exposure to new surfaces (e.g., shaking) but is resistant to thermal denaturation (84.5C).
Ovotransferrin
• A major avian egg white protein (12% of total egg white protein)
• A monomeric glycoprotein consists of a 686‐residue single polypeptide chain. Molecular mass: ~ 78 kDa
• pI: 6.73 for apo‐form (iron‐free), 5.78 for holo‐form
• Possess antiviral and antibacterial activities
• Transport of iron in a soluble form to target cells
• Iron binding protein (reversible, two iron atoms/molecule, as Fe3+).
• The most heat liable in white: forming aggregation by heating at 60 C, resulting in milky white gel
• The release of Fe3+ from ferric transferrin requires the presence of a simple anion such as pyrophosphate, sulfate, and chloride
Ovomucoid
• Best known for its trypsin inhibitory activity
• Hen ovomucoid has nine disulfides and no free sulfhydryl groups
• Heat resistant glycoprotein
• MW is about 28,000, and 11% of total albumen
• Ovomucoid can be heated at 100 C under acidic conditions for long periods without any apparent changes in its physical or chemical properties
• Ovomucoid may play an important role in the pathogenesis of allergic reactions to egg white than other egg white proteins
• Water‐soluble glycoprotein which is antigenic even in boiled shell eggs
• Low ovomucoid egg white preparation
– Applicable as a new processed food for ovomucoid‐sensitive patients.
– A chemically‐altered ovomucoid with enhanced digestibility and lower allergenicity
– Ethanol precipitation
• The disulfide bonds play a significant role in the digestive resistance of ovomucoid
Lysozyme
• Lysozyme constitutes approximately 3.5% of hen egg white
• The name lysozyme was originally used to describe an enzyme which had lytic action against bacterial cells
• Also known as muramidase and N‐acetylmuramic‐hydrolase and is one of the oldest egg components to be utilised commercially
• A bacteriolytic enzyme commonly found in nature and is present in almost all secreted body fluids and tissues of humans and animals.
Egg white lysozyme consists of 129 amino acid residues with a MW of 14.4 kDa
• Binds to ovomucin, transferrin or ovalbumin in egg white
• Highly stable in acidic solution and heating at 100 C for 1‐2 minutes
• The thermal stability of lysozyme is partly due to its four disulfide bonds
• Catalyzes the hydrolysis of the (1‐4)‐glycosidic linkage between N‐acetylmuraminic acid and N‐acetylglucosamine in the polysaccharides of certain bacterial cell walls
Its enzyme activity can be enhanced by certain substances including
• EDTA
• Butylparaben
• Tripolyphosphate
• other naturally occurring antimicrobial agents
Use of Lysozyme
• Pharmaceutical industry: against bacterial, viral or inflammatory diseases such as dental caries and spray for nasal tissue protection
• Therapeutic creams: protection of the skin and soft tissues (e.g. burns, viral diseases).
• Oral administration: shown to have an antihistaminic effect.
• In cheese making: prevent off‐flavours and late blowing in some cheeses (e.g. Swiss Cheese, Parmesan, Edam, Gouda and Cheddar).
• Acceleration of cheese ripening: lysis of starter bacteria releases cytoplasmic enzymes, which play a key role in proteolysis during cheese ripening
• Brewing: control of lactic acid bacteria in beer.
• Sulfur dioxide (SO2) is commonly used to inhibit spoilage bacteria and yeasts in wines, but may cause allergic reactions in sensitive individuals.
• The high affinity binding of lysozyme to bacterial lipopolysaccharide results in reversible inactivation of its enzymatic activity
• Lipophilization broadened the bactericidal action of lysozyme to Gram‐negative bacteria with little loss of enzymic activity
• Glycosylation produces more stable proteins, with improved conformational stability, protease resistance, charge effects and water‐binding capacity
• Lysozyme‐dextran conjugate: retains good emulsifying properties and heat stability
• Heat denaturation of lysozyme results in the progressive loss of enzymatic activity, but a greatly improved antimicrobial action towards Gram‐negative bacteria.
Why lysozyme has been used as an antimicrobial agent in various foods
• Heat stable
• Active in a broad range of temperatures (from 1 C to nearly 100 C)
• Withstands boiling for 1‐2 min
• Stable in freeze‐drying and thermal drying
• Not inactivated by solvents,
• Maintains its activity when redissolved in water
• Has optimum activity at pH 5.3 to 6.4 (i.e. typical for low‐acidic food)
• The presence of other proteins in food, however, can reduce its
stability by the formation of sulfide bridges
Ovomucin
• Ovomucin comprises 1.5‐3.5% of the total egg white solids
• Consists of an a‐subunit (220 kDa containing 10‐15% carbohydrate) and a β‐subunit (400 kDa containing 50‐65% carbohydrate), which are bound by disulfide bonds
– The β‐subunit from ovomucin was shown to have a cytotoxic effect on the cultured tumour cells through scanning electron microscopy
– Both fragments of highly glycosylated peptide fragments (220and 120 kDa) separated from pronase‐treated hen egg white ovomucin derived from the β‐subunit inhibited the growth of tumours
A glycoprotein that contributes the gel‐like structure of thick white
• Heat resistant
• The amount in thick albumen is 4x higher than that in thin white
• Thinning of thick albumen is caused partly by the interaction of ovomucin with lysozyme when the pH rises to around 9.0.
• The formation of ovomucin with lysozyme complex causes the loss of lysozyme activity.
• Inhibits hemagglutination by virus
Avidin
• Avidin is a trace component (0.05%) of egg white
• A tetrameric, strongly basic glycoprotein protein
• Composed of subunits of identical amino acid composition and sequence (15.6 kDa and 128 amino acids each)
• Combines with biotin to form a stable complex, which is incapable of absorption by the intestinal tracts of animals.
• Avidin binds with 4 biotin molecules.
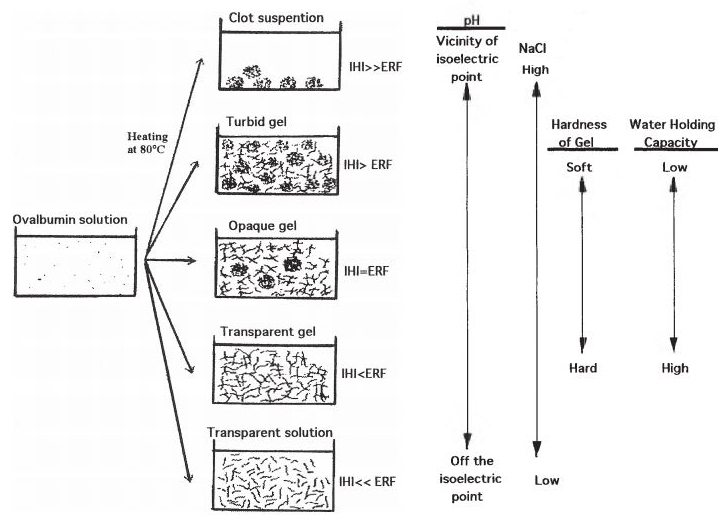

|
|