향기물질 : Damascenone, 장미, 맥주, 와인
Fun ≫ Flavor ≫ 향료물질
향기물질 : Damascenone
- 향기물질 : 베타-다마세논
- 장미이야기, 장미오일
- 맥주, 와인
- 수박, 토마토
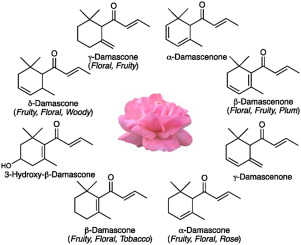
Damascenone 는 장미(Rosa damascene) 이름에서 유래했다. 50년전 Kováts는 불가리아의 장미오일을 분석하여 이것이 주 향기물질임을 밝혔다.
Beta-Damascenone은 매우 작은 양으로 감지 가능하다. 장미오일에서 함량은 시트로넬롤(38%)의 1/270이지만 1/4400의 작은 양으로 감각이 가능해 장미향에 기여도가 16배나 높다.
알코올과 혼합물에서는 1000배나 잘 감지되어 맥주와 와인에서 가장 중요한 향기물질 중의 하나이다
와인에서 ethyl esters (ethyl cinnamate and ethyl hexanoate)의 향을 상승시켜 과일향을 강화하고, 시키고 3-isobutyl-2-methoxypyrazine향을 감소시켜 허브/채소의 느낌을 마스킹한다. 와인향에 간접적인 영향이 더 큰 것이다
Damascenone takes its name from Rosa damascena, the Damask rose, which does take its name from Damascus – this cultivated rose is believed to originate in the Near East, maybe having been brought back from there at the time of the Crusades. In any case, it is an easier name to remember than 3,5,8-Megastigmatrien-7-one, let alone 1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one.
Some 50 years ago, Kováts, working at ETH Zurich, analysed Bulgarian rose oil (from Rosa damascena), identifying a wide range of molecules present, some in large amounts, some in tiny quantities. However, to adapt an expression of George Orwell’s (Animal Farm), “All molecules are equal, but some are more equal than others”. It is some of the least abundant molecules which have the greatest influence on the smell of roses, as they are molecules which individually have a very intense smell.
A molecule like citronellol makes up over 1/3 of the rose oil studied, but its odour threshold, the level below which it cannot be detected by nose, is quite high (40 parts per billion). Thus molecules like that make a small contribution to the smell of rose oil.
Damascenone, a C-13 norisoprenoid, is an important aroma compound and is universally found in grapes and nearly all wines. It was first characterized as a rose ketone compound1 and has since been described as contributing aromas of honey, fruit, floral, tea, stewed apple, dry plum, dark berries and caramel2-4. In a wine matrix, the odor threshold for damascenone is 4-7 μg/L5. Damascenone is typically found at levels below this threshold in red wines (1-2 μg/L) and at or above this level in white wines (5-10 μg/L). Literature suggests damascenone has an indirect impact on red wine aroma. Damascenone has been shown to increase the threshold of fruity esters (ethyl cinnamate and ethyl caproate) and decrease the odor threshold for IBMP, green bell pepper