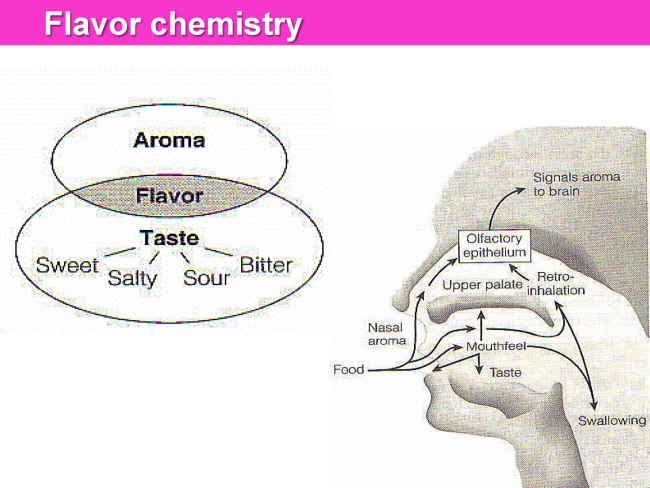
식품 ≫ 즐거움 ≫ 의미
향과 맛 분자의 특징
맛의 종류와 특징
- 고분자, 불용성(비휘발) : 무미, 무취
- 중간 ~ : 쓴맛, 지속성
- 친수성 : 감미, 산미, 감칠맛
- 분자가 커질수록 중량당 분자 숫자는 적어진다
- 분자가 커질수록 움직임은 완만해진다
- 분자가 커질수록 늦게 반응한다
- 분자가 커질수록 맛은 느끼기 어렵게 된다
- N,S 함유 물질은 향의 강도가 강하다
- 저분자 일때는 악취가 되고 고분자가 되면 향이 되는 물질이 많다
- 향과 휘발성, 투과성, 결합력
- 맛과 용해도
향기성분 분자량 17~ 300
- 분자량 : 17(암모니아) ~ 300(무스크류)
탄소수 C16이하
- C 짧으면 Top note, C길면 Bottom note : 1개당 2배 시간
- 대부분 C,H,O,N,S로 구성
- 양극성 : 수용성과 지용층을 둘 다 통과 가능 해야 함
방향족(芳香族, Aromatic) 탄화수소가 유리
- 반응성 높은 물질은 배제되어야 함
- 동일한 냄새가나는 다른 분자는 없다
방향족(芳香族) 탄화수소 : 방향족은 지방족에 비해 훨씬 더 강하고 특이한 냄새를 지닌 일련의 탄화수소 족에 붙여졌다. 화학에서 방향족성은 이 계열에 속한 분자들의 전자구조에서 기인된, 특히 반응성이 낮은 화학적 성질을 의미한다.
Aldehydes
Aldehydes are extremely common components of any food or flavouring and many have a low odour threshold. The straight-chain unbranched aldehydes are ubiquitous. Acetaldehyde is a crucial component of many fruit flavourings, imparting fruity ether notes, whereas the C3–C5 aldehydes (propanal, butanal and pentanal) tend to have a rather chemical/malty/green note to them that is hard to define. The branched chain C5 aldehydes, namely, 2- and 3-methylbutanal (1), have low odour thresholds and are found in most cooked foods as well as in fresh fruits and vegetables. They can impart a very chemical note, particularly when assessed by GC-O but, on dilution, provide malty, bitter cocoa notes that are essential for many malty and chocolate aromas. At C6, the aldehydes become characteristically green. Hexanal imparts a greenbean and cut-grass character when assessed alone, and contributes to the fresh green aroma of green beans and green apples for example, as well as the leafy and less ripe notes in many fruit aroma profiles. It has been shown, for example, that hexanal and related C6 aldehydes decrease as nectarine fruit matures on the plant (Engel et al., 1988b). The use of AEDA has shown hexanal amongst the compounds with the highest FD factors found in tomatoes (Mayer et al., 2003), pomegranate (Cadwallader et al., 2010), Pontianak oranges (Fischer et al., 2008) and apples (Fuhrmann and Grosch, 2002) and undoubtedly this is the case for many other fruits and vegetables.
Hexanal plays a major role in sweet cherries (Sun et al., 2010) where it, and the other C6 aldehydes and alcohols, comprise the major components of the volatile profile. Since it is also formed during thermal processing, it is often detected by GC-O in cooked foods. Indeed, there are few instances in the literature where hexanal is not detected by GC-O, having been reported with high FD factors in many food products, including soy milk (Kaneko et al., 2011), Turkish olive oil (Kesen et al., 2013), wild mushroom (Miyazawa et al., 2010) and Beijing roast duck (Chen et al., 2009). As the chain length increases beyond C6, the aldehydes take on a dual character and have both fruity/floral and fatty descriptors, depending on the concentration present and, of course, the individual who is perceiving them. Octanal still has a fruity note with a fatty character, and decanal is characteristically orange with fatty undertones. However, as the chain gets longer, the character becomes, without doubt, fatty (e.g. dodecanal). Aldehydes are very common in all types of fruits, and the longer chain aldehydes, being lipid-derived, are abundant in meats, fish and fried snacks. Isobranched and ante-isobranched aldehydes, for example, 12-methyltridecanal, have been identified as character impact components of boiled beef (Gasser and Grosch, 1988) and were identified as one of the compounds with the highest OAV in stewed beef (Guth and Grosch, 1994). However, in a recombinate, the addition of this compound had little effect on the aroma.
The unsaturated aldehydes, which exist as both E- and Z-isomers, tend to have
lower odour thresholds and are often character impact compounds, with the shorter
chain analogues providing green aromas. Cis-3-hexenal has an odour threshold of
0.25 μg/kg in water (Buttery et al., 1971) and gives a particularly fresh note to tomatoes, but is also important in pomegranate, oranges and apples (as above) as well as freshly picked coriander (Cadwallader et al., 1999) and bay (Kilic et al., 2004). It is readily isomerised to trans-2-hexenal (Chapter 15, Figure 15.2), which is also described as green, but secondary descriptors such as bitter and stink-bug indicate a less clean fresh green aroma, and the odour threshold is about 100 times higher than for the cis-3-isomer. The decline in the perceived freshness of fresh fruit during storage is often attributed to this interconversion. At C7, the aroma starts to become fatty with 2-heptenal being described as green and fatty. The far more powerful isomer, cis4-heptenal, is described as potatoey and also is likened to linseed oil and is very typical of lamb fat. It is odour-active in many food products, particularly meat, but seems to give the characteristic fishy aroma to fish and seafood, including hake (Triqui, 2006), turbot (Prost et al., 1998), grey mullet (Cayhan and Selli, 2011), mussels (Le Guen et al., 2000) and salt-fermented anchovy (Cha et al., 1999). The C9 (Z)-6-nonenal gives a cucumber note to immature Charentais melons (Lignou et al., 2014), whereas from C10 (2-decenal) to C14 (2-tetradecenal), the (E)-2-alkenals are abundant in coriander and give the typical aroma of coriander, particularly (E)-2-undecenal. Branched 2-alkenals are often formed as a result of the aldol condensation between two aldehydes (see Section 8.2.1.3, Chapter 8); for example, 5-methyl-2-isopropyl-2-hexenal (woody, lavender) or 5-methyl-2-phenyl-2-hexenal (2), which is otherwise known as cocoa hexenal and is very important in chocolate aroma. Many aldehydes with two double bonds (i.e. alkadienals) have low odour thresholds. The 2,4-alkadienals are particularly important in fried aromas and have a characteristic fried chip note when assessed by GC-O. (E,E)-2,4-Decadienal (3) imparts a characteristic fried note, although some assessors describe the same note as lemon or citrus. The aroma threshold in water is 0.2 μg/kg (Belitz et al., 2004) and it is also reported to provide the species character in fried chicken (Gasser and Grosch, 1990). The C9 analogue, 2,4-nonadienal, imparts a similar fried note, but a shift in the position of the double bond to (E,Z)-2,6-nonadienal gives the character impact compound responsible for the aroma of cucumber. Lipid-derived aldehydes are discussed in more detail in Chapters 8 and 9. Related closely to the 2,4-alkadienals is a series of trans-4,5-epoxy-(E)-2-alkenals, many of which have been found in food. They all have a metallic odour, but the most potent of these is trans-4,5-epoxy-(E)-2-decenal, which is often reported to be amongst those compounds with high FD factors for example in soy milk (Kaneko et al., 2011), potato chips (Kasuga et al., 2008) and in black tea (Kumazawa et al., 2008) where both cis and trans isomers were reported to impart sweet juicy notes to the black tea infusion. The odour threshold, which is incredibly low (6 x 107 μg/kg), was determined by GC-O by Buettner and Schieberle (2001). Aldehydes containing an aromatic ring such as benzaldehyde (cherry, almond), phenylacetaldehyde (rose, honey) and cinnamaldehyde (4) (cinnamon) are important components of foods and flavourings. The most ubiquitous of all aroma compounds, vanillin, is an aldehyde with the chemical name 4-hydroxy-3-methoxybenzaldehyde (41). Aldehydes very readily react with alcoholic solvents (e.g. ethanol and polyethylene glycol) to form acetals (Elmore et al., 2014). This happens readily at room temperatures, and acetals are abundant in alcoholic beverages, as well as being formed in many alcohol-based flavourings. The aroma of the acetal is often similar to that of the corresponding aldehydes, but they tend to be less potent. However, the reaction is reversible and the equilibrium can be shifted toward the aldehyde in dilute solution.
Alcohols
Alcohols are also abundant in foods and flavourings, but their contribution to aroma
tends to be less than for the aldehydes. The straight-chain alcohols are abundant in fruits, often increasing with maturity. 2-Methyl-1-propanol can be associated with brown apples and bruised fruit, whereas the longer chain alcohols can be very soapy (e.g. 1-nonanol). Introducing a double bond into the chain renders them more interesting from an organoleptic point of view, with cis-3-hexen-1-ol (5) imparting a characteristic green leaf note, 1-octen-3-ol (6) being a character impact compound in mushrooms. Geosmin (7) and 2-methylisoborneol (8) – both bicyclic alcohols – impart earthy, musty notes and have very low odour thresholds. Geosmin gives a characteristic note to beetroot (Murray et al., 1975) and baby corn (Mottram et al., 2011) where its presence is attributed to the action of actinomycetes in the soil in which the baby corn was grown. These compounds are often implicated as taints in drinking water, for example.
Ketones
The straight-chain methylketones, containing one carbonyl group in the 2-position, e.g. 2-heptanone (9), impart both a blue cheese and a fruity pear aroma, whereas 3-octanone produces earthy, mushroom notes. The α-dicarbonyl compounds such as 2,3-butanedione (10) (diacetyl) and 2,3-pentanedione have far lower thresholds and provide buttery, creamy notes in many cooked foods. Some of the more structurally complex ketones have a key role in aroma: For example, (E)-5-methyl-2-hepten4-one (filbertone) is a character impact compound of hazelnuts (Matsui et al., 1998), 6,10-dimethylundeca-5,9-dien-2-one (geranyl acetone) is present in many fruits and imparts a floral rose aroma and 4-(4-hydroxyphenyl)butan-2-one (11) (raspberry ketone) has been isolated from raspberries and imparts a characteristic sweet, raspberry milkshake aroma (Larsen and Poll, 1990). The carotenoid-derived ketones such as β-ionone (also important in raspberries) and β-damascenone (12) provide, respectively, a pippy note in orchard fruits and deep juicy notes in, for example, berries, tomatoes and apples. Both were found to give woody notes to red peppers (Jun and Kim, 2002) and carrots (Buttery and Takeoka, 2013) where linden ether (3,6-dimethyl-2,4,5,7a-tetrahydrobenzofuran) was found to have the highest FD factor.
Esters
Esters are fundamental to the aroma of most fruits, comprising the major proportion of the volatile compounds in, for example, melons, apples, pineapple and strawberries. The most abundant is ethyl acetate (13), present in most ripe or ripening fruits. For example, as strawberries ripen, there is a marked increase in the abundance of acetates, promoted by an increase in the alcohol acyl transferase that brings about the esterification of acyl CoAs and alcohols (Gonzalez et al., 2009). Ethyl esters are major components of fruit aroma, particularly ripe fruit where the production of ethanol has boosted their formation. Ethyl butanoate (14) resembles strawberry aroma whilst ethyl hexanoate is characteristic of fresh pineapple and more tropical fruits. The longer chain ethyl esters become soapy, cheesy and waxy. Some esters can be quite characteristic of specific fruits: 3-methylbutyl acetate (15) is characteristic of pear or pear drops, allyl hexanoate (16) is typically pineapple, cis-3-hexenyl butanoate imparts the green leafy aroma of the parent alcohol, and the C9 esters are important for melon aroma. Esters also contribute to the more delicate aromas found in cured ham (Theron et al., 2010) and some cheeses. Ethyl butanoate (14) and ethyl hexanoate are key odorants in Parmigiano Reggiano (Qian and Reineccius, 2003) and blue cheese (Qian et al., 2002).
Lactones
Lactones are cyclic (or intramolecular) esters that are potent aroma compounds formed from the corresponding hydroxy acid. Those based on a furan ring are γ-lactones (e.g. γ-octalactone (or 4-octanolide) and γ-decalactone (17) (4-decanolide)) and tend to impart peachy, creamy and coconut aromas. Consequently, they are very popular in tropical flavours; for example, γ-decalactone (17) is the major lactone in both peaches and nectarines (Engel et al., 1988a) and has a threshold of 11 μg/kg in water. The odour thresholds of lactones decrease significantly as the number of constituent carbons increases.
The δ-lactones, which are based on a pyran ring, are less odour-active than their
furanyl isomers. Several lactones were identified in an extract of sweet cream butter, of which δ-decalactone (18) had the highest OAV and was believed to contribute to the sweet cream aroma. γ-Nonalactone, δ-decalactone and the two unsaturated lactones (5-hydroxyoct-2-enoic acid lactone and 5-hydroxydec-2-enoic acid lactone) were found to have relatively high OAVs in milk chocolate (Schnermann and Schieberle, 1997), and all but the 5-hydroxydec-2-enoic acid lactone were found in the cocoa that had been used in the production of the chocolate. Jasmine lactone (19) provides a floral petal-like aroma to green tea (Katsuno et al., 2014), and lactones also make a significant contribution to the volatile profile of Bourbon whisky, with δnonalactone having a FD factor of 2048, and cis-3-methyl-4-octanolide (20) (which is also known as whisky lactone) and γ-decalactone (17) also contributing to the aroma.
Carboxylic acids
Short-chain carboxylic acids are pungent compounds. The aroma of 3-methylbutanoic acid (21), for example, can be described as Parmesan cheese, but is also very reminiscent of vomit. Short-chain acids are present in many food products and give characteristic notes to balsamic vinegar (Ugliano et al., 2003) and cheese (Qian and Reineccius, 2003). Acetic, butanoic, hexanoic, octanoic and decanoic acids all had high FD factors in Parmigiano Reggiano (Qian and Reineccius, 2003). The longer chain acids are less intense, imparting creamy or, in the case of octanoic acid, a blue cheese note. However, with a methyl or ethyl substituent, 4-methyloctanoic (22) and 4-methylnonanoic acids are believed to give character species to sheep meat (Sutherland and Ames, 1996) and also to goat cheeses (Le Quere et al., 1996).
Terpenes and terpenoids
Terpenes, terpenoids and sesquiterpenes are major components of essential oils and
are responsible for the characteristic aroma profile of many fruits (particularly citrus), herbs and spices. They are biosynthesised in plants from units of isoprene (C5H10) and can be linear, cyclic or polycyclic; however, those that are responsible for odour tend to contain two or three isoprene units (monoterpenes and sesquiterpenes, respectively). One of the most ubiquitous is limonene (23), which has a weak orangey citrus peel aroma but it is not a powerful odorant. It is more commonly used as a solvent, a cleaning agent or as a starting material for the production of other natural flavour compounds. Other less abundant terpenes such as α-thujene (woody) and sabinene (citrus) are present in fruits and spices, and the sesquiterpenes provide more interesting aromas with α-valencene imparting classic citrus notes and both farnesene and humulene a woody spicy note. Pinene, myrcene and ocimene are major components of basil aroma.
Many terpenes can be readily oxygenated, and these are technically terpenoids, rather than terpenes. The terpenoid alcohols such as nerol and geraniol (which are
cis/trans isomers of each other, respectively) and citronellol and linalool (both existing as two isomers) provide delicate lemon, rose and violet aromas, which are abundant in herbs, spices and fruits and are essential to many flavourings. The closely related terpene aldehydes are also extremely important in fruit flavouring. Citral is popular in the flavour industry and exists as a mixture of the (E)- and (Z)-isomers, which are called geranial (24) and neral (25), respectively. Perillaldehyde has a herbal spicy, cumin, citrus aroma and is often incorporated in flavourings whilst sinensal (26) gives a characteristic orange aroma. Terpineol exists as four different isomers, but the most abundant is α-terpineol. It is present in many fruits, herbs and spices and has been shown to contribute to the aroma profiles of orange (Chen et al., 2012), but being the oxidation product of limonene, it increases during storage (Perez et al., 2004) and can be indicative of flavour
deterioration. ()-Menthol (27) is the most familiar of terpenoids, not only providing the classic minty note, but also activating the cold-sensitive receptors in the oral cavity to produce a cooling effect (Eccles, 1994). L-Carvone is of particular interest because it exists in two enantiomeric forms with different aroma properties. R-()-carvone smells of spearmint (and is extracted from Mentha spicata L.) whilst the S enantiomer resembles caraway, accounting for 50% of the essential oil in caraway seeds
떫은맛
감의 떫은맛을 내는 성분은 가용성 타닌(water-soluble tannin)으로 과실 중에 0.5% 이상 함유될 때 혀를 자극하여 떫은맛을 느끼게 된다. 가용성 타닌의 분자량 크기는 500∼3,000 정도일 때 떫은맛으로서 작용이 효과적이다. 떫은감의 가용성 타닌 분자구조는 대개 포도당에 갈산(gallic acid)이 결합된 형태로 되어 있으며, 이것이 아세트알데히드의 작용으로 flavonoid계 페놀 화합물의 축합된 형태로 되어 불용성 타닌(condensed tannin)으로 변하게 된다. 불용성 타닌은 분자량이 커 혀를 자극할 수 없기 때문에 떫은맛이 느껴지지 않는다
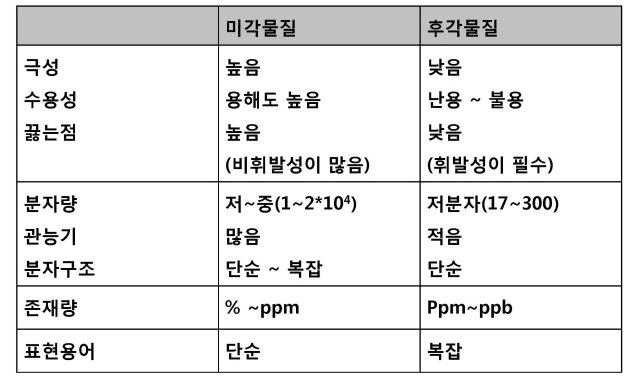
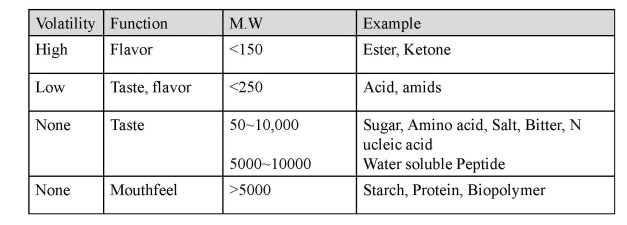

미각 물질의 크기 제한 이유
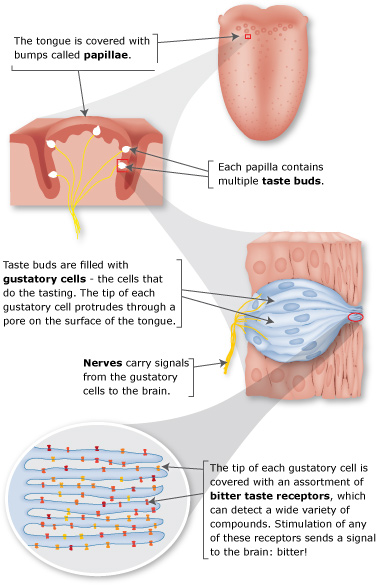
후각물질의 휘발성 이유