| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
원료 ≫ 미네랄 ≫ 인 P 단백질의 인산화 protein phosphorylation 인 P : 미네랄의 여왕 - 인(P)의 생리적 기능 효소 - 슈퍼옥시다제 SOD, 카탈라제 - Fe 함유 효소 - 인산화 Kinase 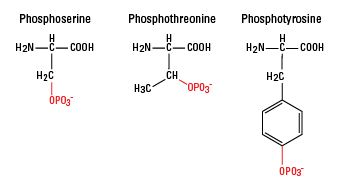  The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review) Authors: Fatima Ardito Michele Giuliani Donatella Perrone Giuseppe Troiano Protein phosphorylation is an important cellular regulatory mechanism as many enzymes and receptors are activated/deactivated by phosphorylation and dephosphorylation events, by means of kinases and phosphatases. In particular, the protein kinases are responsible for cellular transduction signaling and their hyperactivity, malfunction or overexpression can be found in several diseases, mostly tumors. Therefore, it is evident that the use of kinase inhibitors can be valuable for the treatment of cancer. In this review, we discuss the mechanism of action of phosphorylation, with particular attention to the importance of phosphorylation under physiological and pathological conditions. We also discuss the possibility of using kinase inhibitors in the treatment of tumors. 1. Introduction Protein phosphorylation is one of the most common and important post-translational modifications (PTMs) (1,2). This reversible mechanism occurs through protein kinases and consists of the addition of a phosphate group (PO4) to the polar group R of various amino acids. Consequently, this addition modifies the protein from hydrophobic apolar to hydrophilic polar, allowing the protein to change conformation when interacting with other molecules. A phosphorylated amino acid can bind molecules able to interact with other proteins and consequently assemble and detach proteic complexes (3). The interactive capacity of the phosphate group is mainly due to its components. One of its main elements is phosphorus. It has five outer electrons able to form a maximum of five covalent bonds, has three pKas, high water solubility and it can form, for its versatility, mono, di and trialkyl and aryl esters with hydroxyl groups, but also acid anhydrides (4). In particular, many cellular phosphate esters are phosphoproteins that form, via a catalytic enzyme and adenosine triphosphate (ATP), a phosphate anhydride, acting as a donor of a phosphate group. A good energy balance also favors phosphorylation. Indeed, there is a constant balance between phosphorylation and dephosphorylation events mediated by kinases, phosphatases, ATP and/or ADP (protein + ATP ⇄ phosphoprotein + ADP) (5,6) (Fig. 1). Figure 1 Phospho-signaling networks. The mechanism of phosphorylation regulation consists of kinases, phosphatases and their substrates phospho-binding proteins. For example, phosphorylation is activated by stimuli such as epigenetic modifications, cytogenetic alterations, genetic mutations or the tumor micro-environment. Consequently, the protein receives a phosphate group by adenosine triphosphate (ATP) hydrolysis and due to enzymatic activity of kinase. This is the mechanism for the basis of post-translational modification (PTM) formation. In addition, phosphorylation is a reversible process due to activity of phosphatase. Phosphorylation and dephosphorylation are a molecular switch and, in particular, a PTM can cause oncogenic pathway activation by a phospho-binding protein that bind to the phosphate group of a phosphoprotein. The Cell Signaling Technology PhosphoSitePlus (www.phosphosite.org) and the Kinexus PhosphoNET (www.phosphonet.ca) websites both list over 200,000 known human phosphosites, and the Kinexus website predicts another 760,000 additional sites that are likely to be phosphorylated. More than two-thirds of the 21,000 proteins encoded by the human genome has been shown to be phosphorylated, and it is likely that more than 90% are actually subjected to this type of PTM. More than one-third of the protein phosphorylation events occurs on serine (Ser or S), threonine (Thr or T), and tyrosine residues (Tyr or Y) (O-phosphorylation) (7). In particular, the phosphorylated residues of serine are 86.4%, followed by residues of threonine 11.8% whereas only 1.8% of tyrosine residues are phosphorylated (8,9). Tyrosine phosphorylation is relatively rare compared to the other PTMs and is typical of the epidermal growth factor receptor (EGFR) family, which owns a domain called, precisely, tyrosine kinase. Sometimes, phosphorylation of histidine (His or H) and aspartate residues (Asp or D) (N-phosphorylation) also occurs, but, in both cases, this phosphorylation is less stable than others. Protein phosphorylation is a mechanism of regulation that is extremely important in most cellular processes such as protein synthesis, cell division, signal transduction, cell growth, development and aging as many enzymes and receptors are activated and deactivated via phosphorylation/dephosphorylation events due to specific kinases and phosphatases (10). The human genome, in fact, includes approximately 568 protein kinases and 156 protein phosphatases that regulate phosphorylation events and, therefore, play an important role in the control of biological processes such as proliferation, differentiation and apoptosis. For instance, p53 protein is activated by phosphorylation and is then able to stimulate transcription of genes to inhibit the cell cycle, activate DNA repair and in some cases lead to apoptosis (13). An imbalance in the mechanism of phosphorylation/dephosphorylation of the p53 protein can lead to a chronic inactivation of the protein itself, which in turn can transform the cell into a cancer cell. 2. Protein kinases The protein kinases belong to the great family of kinases and are responsible for the mechanism of phosphorylation. They are activated by phosphorylation which in turn activates a cascade of events leading to the phosphorylation of different amino acids (3). Activation or deactivation of kinase occurs in different ways: through the kinase itself with a cis-phosphorylation/autophosphorylation, by binding with activator or inhibitor proteins or checking their localization in the cell in relation to their substrate (7). The catalytic domain of protein kinase has 2 subdomains, N- and C-terminal (8). Both are connected by a peptidic stand, which forms an active site with a front pocket (catalytic residues) and a back pocket. Access to the rear pocket is controlled by a conserved lysine residue and a residue 'gatekeeper'. The catalytic domain is unavailable when it is active because propellers of the N- and C-terminal subdomains rotate inward. The activation of the catalytic domain occurs through phosphorylation of the activation loop or through an allosteric mechanism (8). Moreover, the kinases also have non-catalytic domains allowing the attachment of substrates and the recruitment of other signaling proteins (9). Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction (10). In the last few years, kinases have been considered important not only for their crucial role in signaling but also for the transduction of the signal, controlling its amplitude (11–13). To facilitate the study of phospho-signaling networks, different databases have been designed (2,14,15). The 518 human protein kinases are classified according to the amino acid residue that it phosphorylates. Most kinases act on both serine and threonine (serine/threonine kinases; STKs), others act on tyrosine (tyrosine kinases; TKs), and a number act on all three (dual-specificity kinases; DSKs) (16). The latter can phosphorylate STKs and TKs (17); at least 125 of the human protein kinases are STKs (18). The STKs are enzymes that phosphorylate the OH group of serine or threonine, and are activated by different events such as DNA damage or chemical signals mediated for instance by Ca2+/calmodulin, cyclic-adenosine monophosphate/cyclic-guanosine monophosphate (cAMP/cGMP) and diacylglycerol. 3. Protein phosphatases Phosphatases have the opposite function of kinases. They remove the phosphate group from phosphoproteins by hydrolyzing phosphoric acid monoesters into a phosphate group and a molecule with a free hydroxyl group (28,29). Enzymatic removal reverts the protein to a non-phosphorylated state with a kinetics more rapid than kinases (30). When working with proteins in the laboratory, phosphatases are inactivated using denaturation or inhibitors so phosphorylation inside of a sample is not lost (31). The protein phosphatases are considered passive housekeeping enzymes compared with protein kinases; their different structure makes them harder to identify and less important than the protein kinases (32). Currently, there are approximately 226 known protein phosphatases (33) which are classified into 3 families: phosphoprotein phosphatase (PPP) family, metallo-dependent protein phosphatase (PPM) family and protein-tyrosine phosphatase (PTP) family (19). The PPP family includes PP1, PP2A, PP2B and PP4–7 responsible for many dephosphorylation reactions (1) whereas PP2C is one of the most important members of the PPM family (34). PPP and PMP groups are responsible for most dephosphorylation reactions of phosphoserine and phosphotreonine (pSer/pThr) and they can also dephosphorylate phosphotyrosine (pTyr) (35,36). However, pSer/pThr have different domain sequences compared with pTyr (37). In contrast, the phosphatases that belong to the PTP family have the same catalytic domain but different selectivity for phosphorylated proteins (38,39). Of all the phosphatases, at least 100 belong to those that dephosphorylate tyrosine residues, such as the Tyr-specific phosphatase subfamily, the Cdc25 family and myotubularin-related phosphatase and low molecular weight Tyr phosphatase (12,14,32,33,39,40). Aspartate-based phosphatases, such as FCP/SCP (small CTD phosphatase) (41–43) and TAD (haloacid dehalogenase) family enzymes are part of the PTP group (44). PTPs are well-known as they can also dephosphorylate non-protein targets such as carbohydrates, mRNA and phosphoinositides (12,45–47). 4. Activities and role of protein phosphorylation under physiological conditions Protein phosphorylation is one of the initial steps that is vital for the coordination of cellular and organic functions such as the regulation of metabolism, proliferation, apoptosis, subcellular trafficking, inflammation, and other important physiological processes. There are several ways in which the phosphorylation acts to fulfill its role. First, the activity of phosphorylation/dephosphorylation acts as a molecular switch (Fig. 1). For instance, protein kinase B is only activated follo wing phosphorylation of its Ser and Thr residues and, thus, is able to regulate cell survival (48); on the other hand, when proto-oncogene tyrosine-protein kinase (c-Src) is dephosphorylated, it is turned off causing a block in the regulation of cell growth (49). Another mode of phosphorylation is temporary protein-protein interaction, which allows the adjustment of many signaling pathways (50,51). An example is glomerular podocyte protein nephrin 1 (Neph1), an important protein of renal cells, which once phosphorylated by Src Fyn, interacts with Grb2, an adapter protein involved in signal transduction and cell communication (52). In addition, the phosphorylation of a protein can regulate the process of signal transduction since it is able to trigger the subcellular translocation of the protein phosphorylated by the mechanism itself. Moreover, phosphorylation on serine/threonine-protein kinase (Ser350) residue of the death-associated protein (DAP) leads to the translocation from the cytoplasm to the nucleus of apoptosis-inducing kinase 2 (DRAK2) which is able to induce apoptosis in T and B cells (53). Another example is the membrane translocation of synaptosomal-associated protein 25 (SNAP25). After being phosphorylated, SNAP25 has a reduced binding affinity for syntaxin-1A and thus changes location (54,55). Furthermore, phosphorylation is mainly involved in the production and recycling of ATP and, therefore, is important in biological reactions that require energy (5,56). Sometimes, protein phosphorylation may promote the formation or removal of a second PTM (4,57) (Fig. 1). An example is the phosphorylation of insulin receptor substrate-1 (IRS-1), a mediator of insulin signaling pathway, by the ribosomal protein S6 kinase β1 that induces polyubiquitination of IRS-1 due to E3 ligase-CUL7 and its successive proteasomal degradation (58). The processes of phosphorylation and dephosphorylation can be etremely complex, since a single kinase or phosphatase may simultaneously have more substrates and may function in various cell signaling pathways. One of the signaling pathways known for this function is mitogen activated protein kinase (MAPK/ERK). It is activated through phosphorylation of MAPK which in turn phosphorylates many substrates, including 40S ribosomal protein S6 kinase (RSK, which then phosphorylates ribosomal protein S6), c-Myc and MNK (which then phosphorylates CREB in this cascade) (59,60). MAPK is a known protein involved in a signaling pathway activated by a cascade effect of phosphorylation events (61). The binding of interferon-γ (IFN-γ) to its receptor induces phosphorylation of the Tyr-440 receptor, which promotes the formation of a complex with the tyrosine kinase JAK1 and JAK2. This complex phosphorylates Stat1, leading to its dimerization and nuclear translocation, where it regulates gene transcription (62–65). Therefore, phospho-signaling networks represent the basis of many cellular processes. They consist mainly of protein kinases, phosphatases, and their respective substrates phospho-binding proteins (66) (Fig. 1). There is also a mechanism of competition for kinases and phosphatases at the level of protein sites to adjust the states of phosphorylation of common substrates (67,68). PhosphoNET and PhosphoSitePlus websites document the inhibition or activation of human protein with more than 850 different phosphosites with predictions for over 1,000 additional sites. Therefore, it is customary to classify phosphorylation into two categories: one refers to functional changes (stable) and the other one, transitory, has no effect on regulatory functions. For this reason, it is thought that all stable phosphosites are functional and those not stable, are not functional (69–71). In addition, the functional effects of phosphosites within a protein are site-dependent (72), and this means that they are functional only if phosphorylation takes place on a specific site and not random. This endorses the view that the detailed study of phosphorylation networks may help to understand the physiological and pathological mechanisms (72–76). 5. Protein phosphorylation and cancer Phosphorylation is one of the most common PTMs involved in the regulation of multiple biological processes and overexpression of kinase. Mutations or defects in regulatory mechanisms can lead to aberrant activation or dysregulation of kinase signaling pathways (77) and this is the basis of oncogenesis for multiple tumors (78–80). Cancer is not only considered a disease that arises from genetic mutations, but also a disease that results from epigenetic changes (81–83) that mainly lead to a deregulation of signal transduction pathways with subsequent changes in normal cellular mechanisms (84). Many key regulatory proteins controlling gene expression are targets of kinases. The addition of a phosphate group to a protein by a kinase can alter the activity of the protein and this action is often exploited as a switch on or off (85,86). In chronic myeloid leukemia, a particular chromosomal translocation (Philadelphia chromosome) was identified that generates a novel kinase that is always active, the retinoblastoma, pRb. The process normally controlled by this kinase is stuck in the 'on' position. This leads to the proliferation of tumor cells (87). D. Stehelin was one of the first researchers to understand the direct involvement of protein kinases in tumors, with the study of the oncogene v-SRC (88). This tyrosine kinase with the phosphate group of Tyr-527 has a key role in tumor cell proliferation, and has been studied extensively in Rous sarcoma virus (RSV) as the main cause of sarcoma in chickens (89–92). Its carcinogenic action is due to a mutation of the carboxyl terminal of the molecule able to eliminate the tyrosine residue, which causes conformational changes and also an irregular unregulated autophosphorylation, leading to a signal of increased growth (93,94). Aberrations of kinases have been reported in different types of cancer. An example is the amplification of Her2/neu observed in tumor cells of invasive breast cancer (95,96). Phosphorylation plays a key role even in oral cancer. In fact, the phosphorylation of EGFR (Fig. 2) is important for transactivation of interleukin (IL)-1β through the CXCL1 and CXCR2 axis (97). Figure 2 Endothelial growth factor receptor (EGFR) signaling. Ligand binding to the EGFR activates its intrinsic tyrosine kinase activity. The autophosphorylation of EGFR causes the activation of several signaling pathways such as PI3K/AKT and RAS/mitogen-activated protein kinase (MAPK). In both, the phosphorylation is a predominant event and plays an important role in cell survival (AKT activation phosphorylates BAD and MDM2), cell proliferation (AKT activation phosphorylates FoxO, RAS phosphorylates RAF and it phosphorylates MEK that phosphorylates Erk1/2), cell migration (phosphorylation cascade of RAS), apoptosis (AKT phosphorylation is able to activate caspase). However, the phosphorylation of AKT causes mTOR activation, important in protein synthesis. Alteration of the phosphoproteome also affects gastrointestinal stromal tumors (GISTs) (98,99), lung cancer (73,100), hematologic malignancies (101,102), breast cancer (103,104), pancreatic cancer (105,106) and prostate cancer (103,107). To date, more than 1,000 variations in the expression of protein kinases have been detected in human tumors (108–111). Many of these kinases are now considered cancer biomarkers, such as EGFR for colon cancer, cKIT for GISTs, and human EGFR-related gene (Her2) for breast cancer (112). In tumors, mTOR (Fig. 2) is a kinase and, when activated, is able to induce activation of its downstream effectors and consequently increases the synthesis of cell cycle proteins such as hypoxia-inducible factor-1 (HIF-1) (cyclin D and HIF-1α) which in turn stimulates vascular endothelial growth factor (VEGF) (113,114). mTOR is particularly active in renal cancer by promoting angiogenesis (115,116). The Ras oncogene (Fig. 2) is the most common in human tumors (117) and its activation is very complex, characterized by countless phosphorylation events. It begins with the binding of a ligand to a receptor tyrosine kinase (RTK) loca ted on the plasma membrane. This receptor is activated only if it dimerizes with another RTK. They then phosphorylate each other and become activated. The activated receptor binds to the SH2 domain of the adapter protein Grb2, which plays its role without being phosphorylated. In fact, its SH3 domain binds to the protein activating SOS, without any phosphorylation reactions. SOS then moves close to the plasma membrane where it can bind Ras, replacing GDP with GTP and then becomes activated; SOS therefore acts as a nucleotide exchange factor (GEF). It is known that activated Ras binds to the N-terminus of a protein Ser/Thr kinase called c-Raf, activating them (118). 6. Protein kinases as drug targets The signaling pathways regulated by protein kinases contribute to the onset and progression of almost all types of cancer. Consequently, research of the signaling pathways mediated by kinase and therefore the possibility of blocking them with targeted treatment could have major clinical-therapeutic utility especially since many of these proteins act as oncogenes (78,119,120). Considerable advances have led to the identification of inhibitors directed against activated tyrosine kinases in cancer, 17 of which are already used for the treatment of several cancers and more than 390 molecules are being tested (121). Imatinib (Glivec®) is a known inhibitor that blocks the action of the tyrosine kinase BCR-Abl in patients with chronic myeloid leukemia (CML) (122,123). This drug also targets against PI3K in solid tumors (124,125), serine/threonine kinase BRAF to treat melanoma (126–128), the receptor tyrosine kinase EGFR for lung cancer (129,130), and serine/threonine kinase mTOR for the treatment of renal tumors (131). Gefitinib/erlotinib (Tarceva®) is targeted against EGFR in lung tumors (132) with a success rate of 71.2% (129), whereas crizotinib (Xalkori®) acts in the same tumor against EML4-ALK (133). Vemurafenib (Zelboraf®) in melanoma is directed against mutations of BRAF V600E (134) with a successful response of 48% during treatment (127). If overexpressed, HER2 is a protein tyrosine kinase which enhances the proliferation of cancer cells, and enhances the formation of blood vessels thereby increasing the invasiveness of breast cancer. Currently, improvements in the prognosis of this cancer are due to the use of trastuzumab (Herceptin®) a monoclonal antibody targeted against this protein (135). Sorafenib (Nexavar®) is another kinase inhibitor which blocks the action of Raf kinase in kidney and liver tumors (136). Sunitinib (Sutent®) is a multi-targeted receptor tyrosine kinase inhibitor that acts against platelet-derived growth factor receptors (PDGFRs) and VEGF receptors (VEGFRs) (137). The simultaneous inhibition of these targets induces a reduction in tumor vascularization and triggers cancer cell apoptosis. It has been recommended as a drug in renal cell carcinoma and in GISTs (138,139). Furthermore, since sunitinib targets many different receptors, it leads to dermatologic toxic side effects such as hand-foot syndrome (140). Temsirolimus (Torisel®) is a drug used for the treatment of renal cell carcinoma and it is a specific inhibitor of mTOR (141), a cellular kinase enzyme that may favor tumor growth. Temsirolimus leads to cell cycle arrest in the G1 phase, and also inhibits tumor angiogenesis by reducing synthesis of VEGF (142). The success of therapies based on kinase inhibitors relies on different aspects: the clinical targeted kinase, the structure of the signaling network and the mechanisms of innate or acquired resistance. First of all, both the patients and the therapeutic approach functions must be appropriately selected (119). For instance, in CML, this therapy only works with BCR-Abl-positive patients as the main targets of therapy are BCR and ABL (Philadelphia chromosome genes) fused together by means of an activated protein tyrosine kinase. In the same way, the response rate of Herceptin was 34% in patients whose tumors had amplified HER2 compared with 7% in those whose tumors did not (143). However, not all tumors respond to inhibitors of kinases and often patients with the same cancer respond differently to the same therapy. For this reason, patients should be further stratified using biomarkers (144) and further studies are warranted to investigate the signaling pathways (145–147). In this respect, we know that changes in the signaling pathways, caused by several factors (genetic and epigenetic mutations, alterations of the microenvironment), lead to the formation of oncogenes and, very often, there is a release of tumoral molecules that can be tracked and used as biomarkers. For example, hepatocyte growth factor (HGF) released in stromal cells of melanoma, influences the way BRAF inhibitors act (148). The signaling networks of cancer cells can also develop innate or acquired resistance, since they are able to create the most common or rare oncogenic mutations different from tumor to tumor (the so-called polygenic tumor biology) (149). There are two main types of resistance to a drug treatment based on kinase inhibitors. Intrinsic or innate resistance (on target) occurs when the drug target protein has changed due to steric hindrance to inhibitor binding (150), altered active site topography (151), disruption of favorable inhibitor interactions (152), altered protein dynamics (153), increased oncogenicity (151), and alteration of ATP affinity (154). In this way, this resistance is not inhibited by the drug and continues to perform its normal activity in the tumor cell. In extrinsic or acquired resistance (off target), the signaling network is able to restore the function of oncogenic mutation in the signaling network (155,156) or via bypass/compensatory signaling or even through feedback loops to adjust the signal. The cancer cells are able in fact to exploit and reactivate the mechanism of signaling that the drug would inhibit (157). In addition, during treatment acquired resistance can occur and the tumors can develop subclones which foster even relapse. New studies of the signaling network of tumors with particular attention to the mechanism of action of drug inhibitors of protein kinases are therefore needed. 7. Conclusions Phosphoproteomics has a critical relevance for many aspects of biology and has a significant role for understanding the molecular mechanisms, especially those that lead to the genesis and growth of tumors (77–79). Signaling networks in which protein kinases operate are highly complex, but we believe that understanding the regulatory functions of kinases may be a valid means to identify more effective therapies against cancer (146,147). Many drug kinase inhibitors are already on the market (122,133–137) but, often, their effectiveness is reduced due to the development of complex mechanisms of drug resistance (149). However, great progress has been made in recent years thanks to the numerous techniques of proteomics. Proteomics is the most important way by which to study the sites and behavior of phosphoprotein and phosphosite in tumor biology. The identification of biomarkers that aid in the selection of the most appropriate therapy for individual patients remains a major challenge (144). |
||||
|
|
|||