| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
암 ≫ 오해 ≫ 발암물질 산화 에틸렌(ethylene oxide) 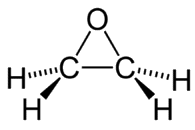 상온에서 무색의 기체 상태로 존재한다. 녹는점은 -111.3°C, 끓는점은 13.5°C이다. 액체 상태에서의 비중은 0.887이다. 물또는 알코올과는 임의의 비율로 섞일 수 있으며 에테르에도 잘 녹는다. 물이 혼합되어 있을 경우 분해하여 에틸렌 글리콜이 된다. 환상의 에테르 구조를 갖는 상온에서 무색의 기체로 특유의 향기를 갖는다. 산화에틸렌이라고도 한다. 끓는점은 10.8℃이고, 반응성이 풍부하고 공업적으로는 에틸렌을 산소로 산화하여 합성한다. 반응성이 풍부하고, 폭발성이 있다. 물이나 묽은 황산과 작용하면 glycol을, 수소로 환원하면 에틸알코올을, 암모니아와 반응하면 아미노알코올을 생성한다. Ethylene glycol, polyethylene glycol 또는 그들의 유도체의 합성에 많이 이용되고 있다. 각종 화합물과의 반응성이 높고, 핵산 등과도 반응하여, 강한 살균력이나 살충력을 보이고 의료기구나 포장용기의 가스살균제로서 쓰이고 있다. Ethylene oxide 가스는 강한 독성을 보이고, 잔류성이 크다. 잔류한 ethylene oxide는 각종화합물과 반응하여 glycol을, 식염과 반응하여 chlorohydrin을 각각 생성한다. 이러한 이유에 의해 우리나라에서는 ethylene oxide를 식품의 처리에 사용하는 것은 금지되어 있다. 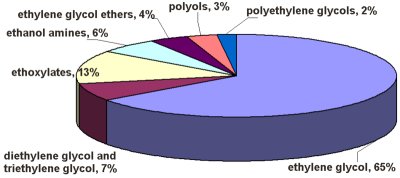 Ethylene oxide is one of the most important raw materials used in the large-scale chemical production. Most ethylene oxide is used for synthesis of ethylene glycols, including diethylene glycol and triethylene glycol, that accounts for up to 75% of global consumption. Other important products include ethylene glycol ethers, ethanolamines and ethoxylates. Among glycols, ethylene glycol is used as antifreeze, in the production of polyester and polyethylene terephthalate (PET – raw material for plastic bottles), liquid coolants and solvents. Polyethyleneglycols are used in perfumes, cosmetics, pharmaceuticals, lubricants, paint thinners and plasticizers. Ethylene glycol ethers are part of brake fluids, detergents, solvents, lacquers and paints. Other products of ethylene oxide. Ethanolamines are used in the manufacture of soap and detergents and for purification of natural gas. Ethoxylates are reaction products of ethylene oxide with higher alcohols, acids or amines. They are used in the manufacture of detergents, surfactants, emulsifiers and dispersants Effect on microorganisms Ethylene oxide inhibits growth of microorganisms (disinfectant properties) and when present in high concentrations, can completely destroy them. Strong alkylating properties make ethylene oxide a universal poison for protoplasm: it causes clotting of proteins, deactivation of enzymes and other biologically important components of a living organism. Ethylene oxide acts more strongly against bacteria, especially gram-positive bacteria, than against yeast and fungi.[97] The disinfectant effect of ethylene oxide is similar to that of sterilization by heat, but because of limited penetration, it affects only the surface. The Sterility Assurance Level, after a certain specified exposure to ethylene oxide is 10−6, meaning that the chance of finding a single bacterium is below 1 per million.[98] The median lethal doses (LD50 ) for ethylene oxide are 72 mg/kg (rat, oral) and 187 mg/kg (rat, subcutaneous injection). |
||||
|
|
|||